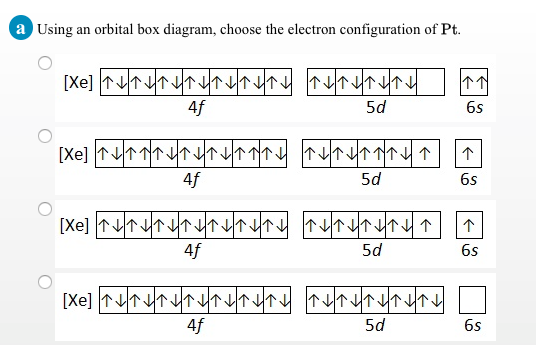
orbital diagram for pt
The electron configuration for helium is 1s². The CN - is a strong field ligand and causes the unpaired.
Principles Of Chem 1
The hybridization is dsp 2 and the electronic configuration of Pt 2 is 5d 8.

. Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below. The electron configuration of ruthenium ion Ru 3 is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 5. Then state how many valence electrons there are for Pt and tell this atom will be paramagnetic or diamagnetic.
The first number is the principal quantum number n and the letter represents the value of l angular momentum. This problem has been solved. The orbital filling diagram for helium.
Electron configurations have the format. The Aufbau principle the Pau. Orbital diagrams Orbital box diagrams of all elements are mentioned in the.
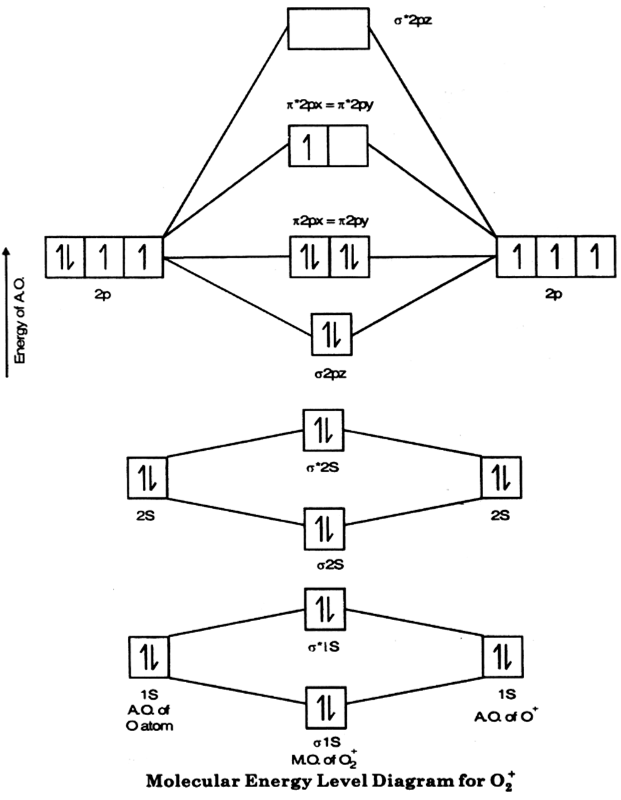
Create a molecular orbital diagram of Pt CN42- by considering the σ orbitals only. Ru 3e Ru 3. Orbital diagrams are a visual way to show where the electrons are located within an atom.
This is clearly shown in the figure of the orbital diagram of palladium. Sulfur excited state electron configuration and orbital diagram. That is the number of electrons in xenon is fifty-four.
Again Mn 4e Mn 4. So the electron configuration of chlorine Cl in an excited state will be 1s 2 2s 2 2p. The platinum metal Pt is in 2 oxidation state.
The sub-level 2s drawn in the 1 energy level there are two electrons in this orbital. According to the Auf Bau Principle each electron occupies the. Xenon atom electron configuration Bohr model The atomic number of xenon is 54.
The orbital diagram will be filled in the same order as described by the Aufbau principle. 1s 2 2s 2 2p 6. This means that we have two electrons in the 1s orbital which looks like this.
The orbitals are d xy d yz d zx d x2-y2 and d z2 and each orbital can have a maximum of two electrons. Therefore the electron configuration of sulfur S in an excited state will be 1s 2 2s 2 2p 6 3s 1 3p x1 3p y1 3p z1 3d. Orbital diagrams are pictorial descriptions of the electrons in an atom.
Write down the electron configuration and the orbital box diagram of. The order in which the orbitals are filled with electrons from lower energy to higher energy is. The sub-level 2p drawn in the energy level 15 ie.
The ground state electron configuration of palladium is 1s 2 2s 2 2p 6. This electron configuration shows that the ruthenium ion Ru 3 has. Orbital diagrams must follow 3 rules.
Three rules are useful in forming orbital diagrams. From the above information we can say that manganese exhibits 2 3 and. Consider CN- as spheres that use σ orbitals to interact with.
The electron configuration of manganese ion Mn 4 is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 3. The 6 electrons will go to the 2p orbital and the next 2 electrons will place with the 3s orbital and now we have only 8 electrons in which 6 electrons will go with the 3p orbital. 1s 2s.
In the diagram the vertical spacing is. Palladium ionPd 2 electron configuration. Therefore a xenon atom will have two electrons in.

Molecular Orbital Diagram For The Model Allyl Complex 2 Ph 3 2 Download Scientific Diagram
Solved Manganese Is Found As Mno2 In Deep Ocean Deposits Chegg Com

Molecular Orbital Theory For Homonuclear Diatomic Molecules Pt 3 Youtube
What Is The Electron Configuration Of Pt 2 According To The Madelung Rule Socratic
Electron Configuration Wyzant Lessons

Palladium Element Information Properties And Applications
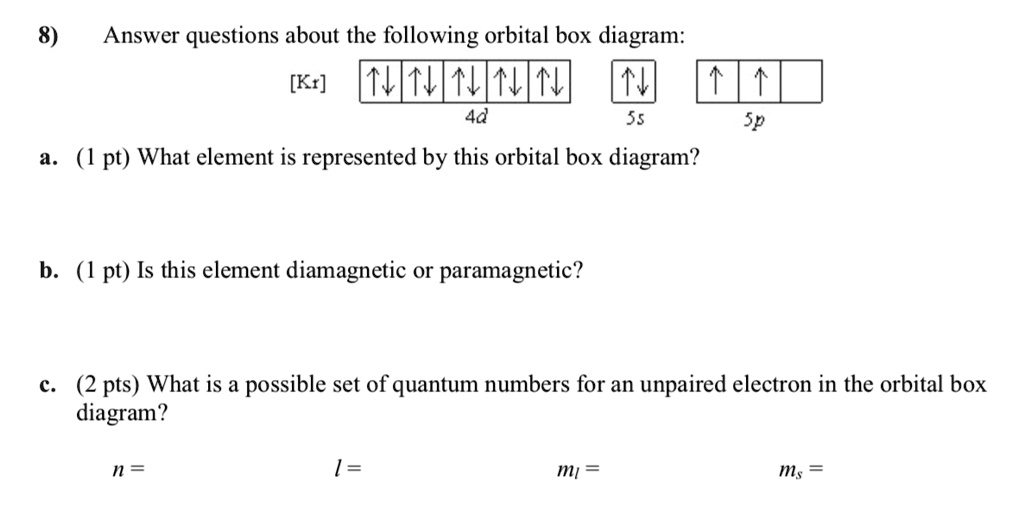
Solved 8 Answer Questions About The Following Orbital Box Diagram Kt Nnv 4d 55 A Pt What Element Is Represented By This Orbital Box Diagram Sp Pt Is This Element Diamagnetic Or

Qualitative Orbital Energy Level Scheme Of Pt 2 4o And Tetrahedral Download Scientific Diagram

Molecular Orbital Diagram For The Complex Ph 3 2 Pt H 3 Chcch 2 Download Scientific Diagram
Two Ionic Compounds Ab Find Ab2 Have The Same Solubility Product Which Of The Two Compounds Has A Higher Solubility At The Same Temperature Justify Your Answer Wired Faculty

A Draw The Molecular Orbital Diagram For Se 2 2 B Write The Molecular Orbital Configuration For Se 2 2 C Is The Molecule Paramagnetic Or Diamagnetic D What Is The Bond Order E Is The
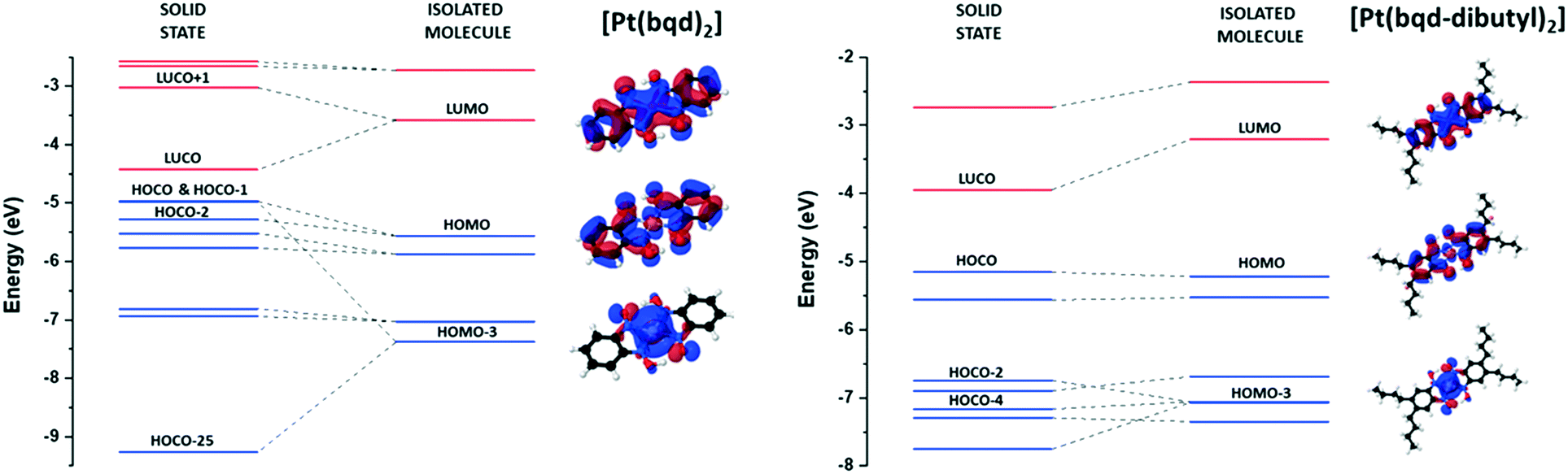
Evaluating The Crystalline Orbital Hierarchy And High Pressure Structure Property Response Of An Extended Ligand Platinum Ii Bis 1 2 Dioximato Com Crystengcomm Rsc Publishing Doi 10 1039 D1ce00892g
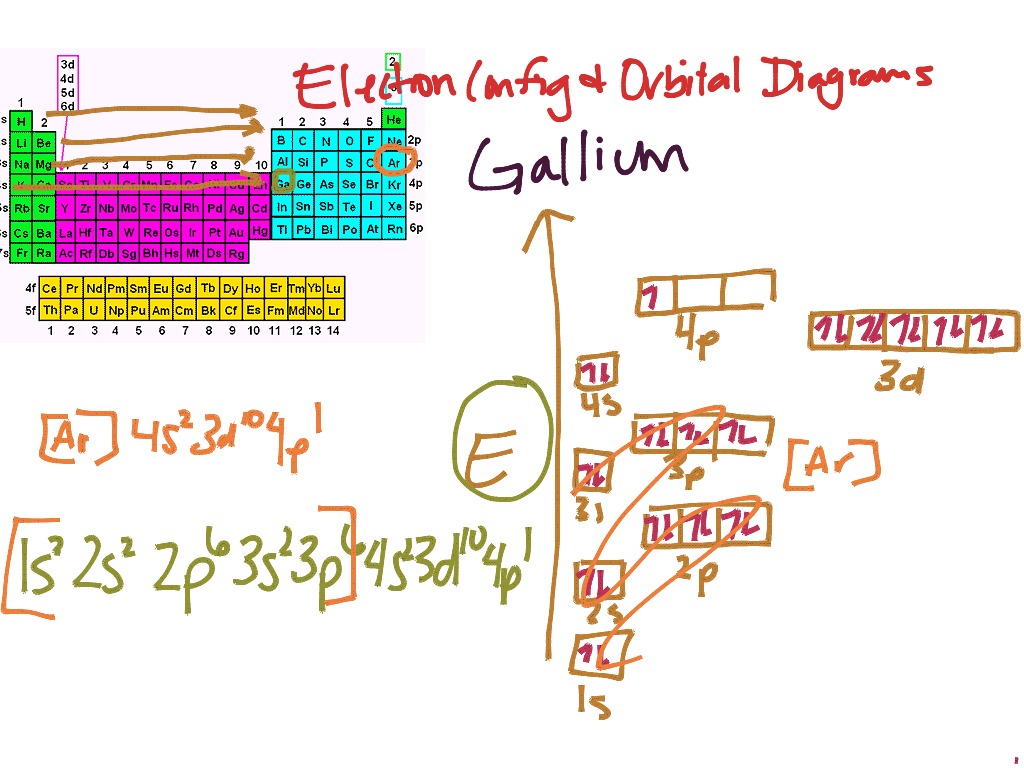
Electron Configuration And Orbital Diagrams Science Chemistry Elements Showme

Electronic Configuration Is The Order Of Orientation Of Electron Box Diagrams Meaningful Or Arbitrary Chemistry Stack Exchange
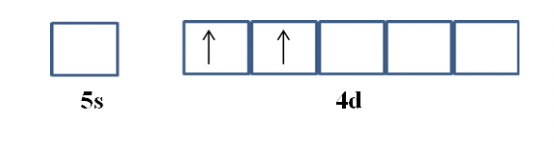
Solved The Partial Valence Level Orbital Diagram For A 2 Chegg Com
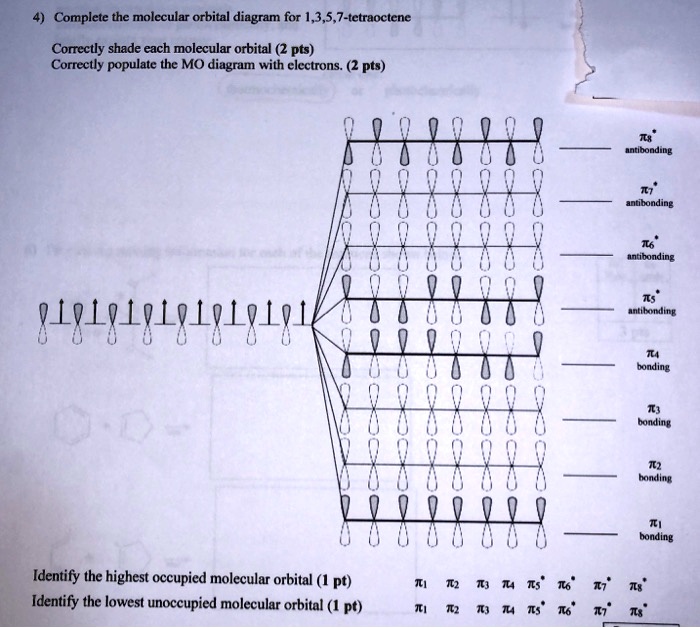
Solved Complete The Molccular Orbital Diagram For 1 3 5 7 Tetraoctene Correctly Shade Each Molecular Orbital 2 Pts Correctly Populate The Mo Diugrum With Electrons 2 Pts Antlbunding Antibonding Antibonding Antikonding 84848184848484848 Bonding

Solved For Se Atomic Number 34 In The Ground State Depict The Completelelectron Configuration 3 Points 0 4 Ls 2 5 3p 3s 30 4j 34 4 P Depict The Complete